Absorption photometry is an analytical technique that measures the amount of light absorbed by a liquid solution, at a specific wavelength, in order to determine the concentration of an absorbing substance. In this technique, a beam of light passes through a solution, and the decrease in light intensity due to absorption is measured.
- About
- Solutions
-
Clinical Analysis
-
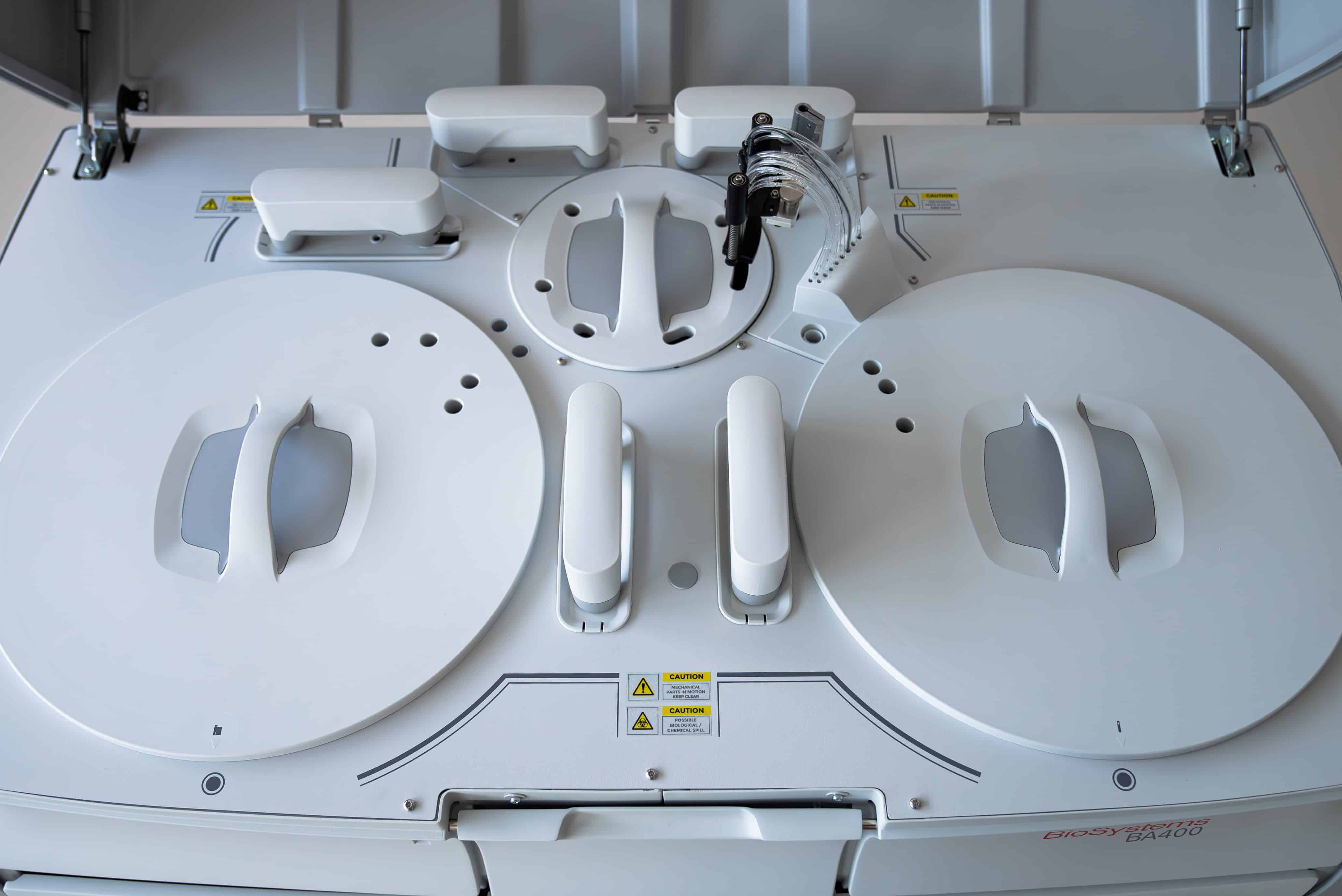
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Autoimmunity Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Veterinary Analysis
-
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Vector-borne Diseases Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Food & Beverage Analysis
-
Environmental Analysis
-
Bioprocess Analysis
-
- Discover
- Contact
- Resources












1.thigh.jpg)


