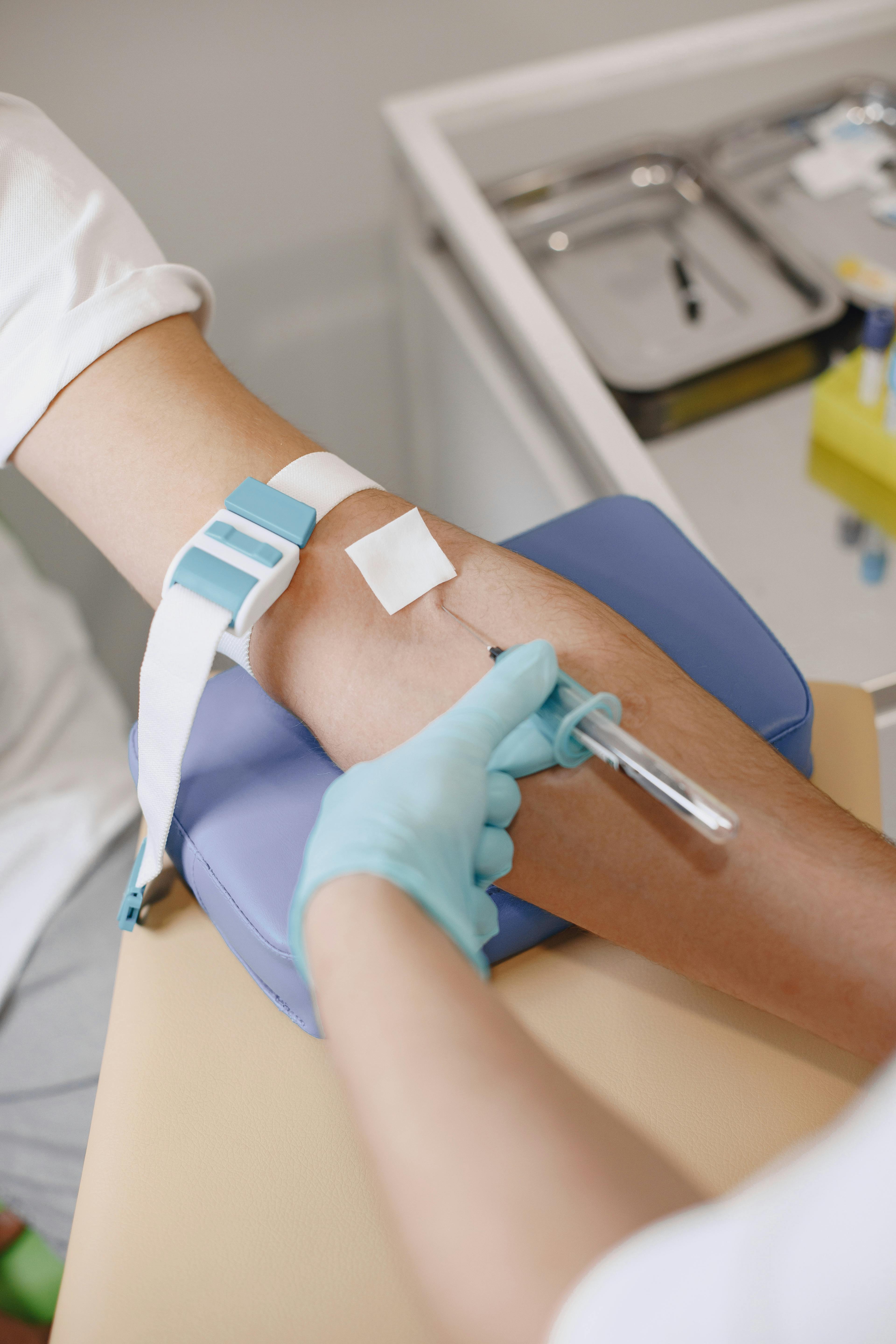
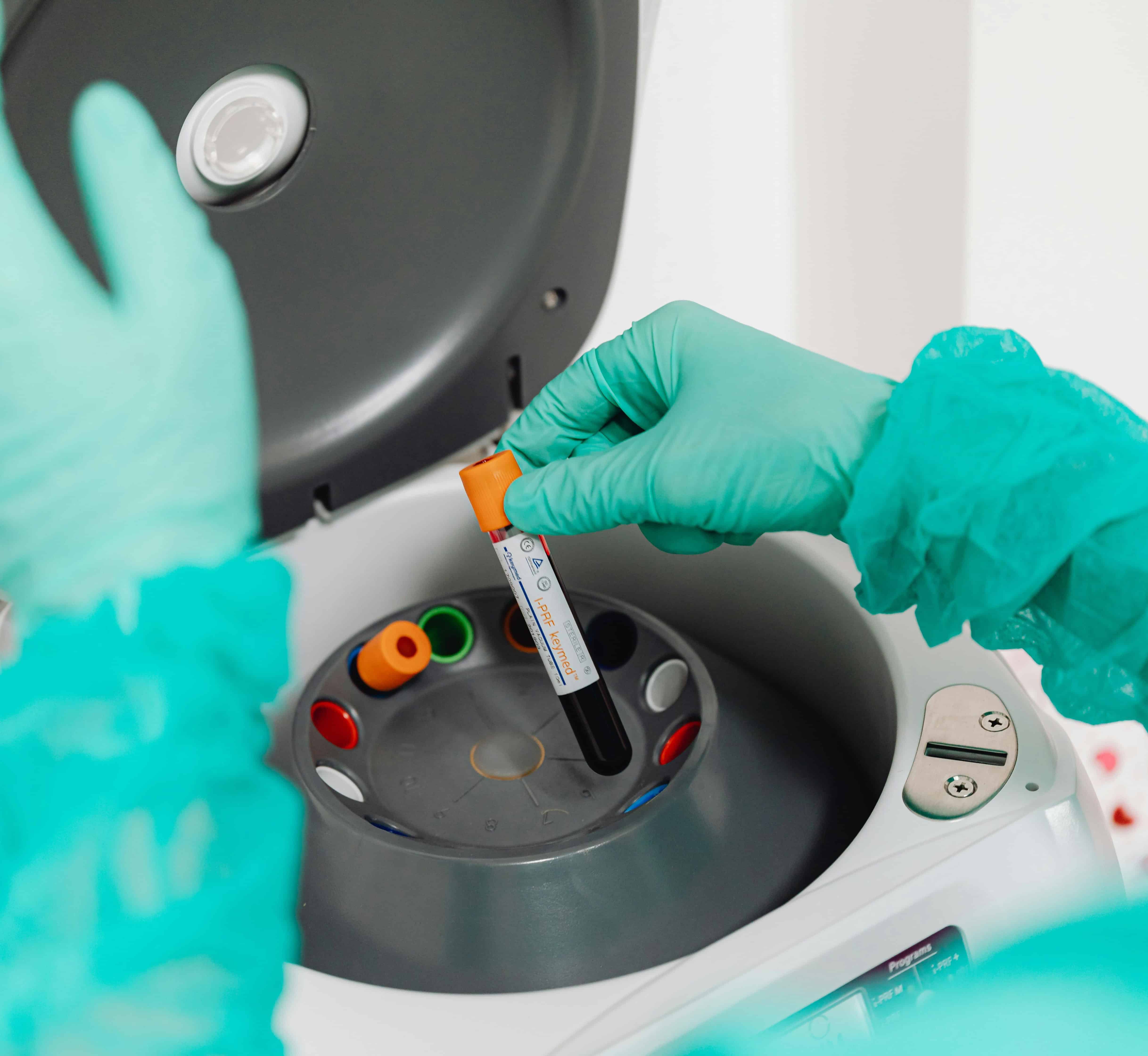
The pre-analytical phase encompasses all steps from the generation of the analysis request to the measurement of the biological magnitude. It includes processes both outside and inside the clinical laboratory. According to several studies, the highest percentage of errors in the clinical laboratory, around 60%, occurs in this phase. Therefore, taking care of it is essential to improve the quality of analyses and patient safety. Let's look at its different steps.
- About
- Solutions
-
Clinical Analysis
-
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Autoimmunity Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Veterinary Analysis
-
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Vector-borne Diseases Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Food & Beverage Analysis
-
Environmental Analysis
-
Bioprocess Analysis
-
- News
- Contact
- Resources