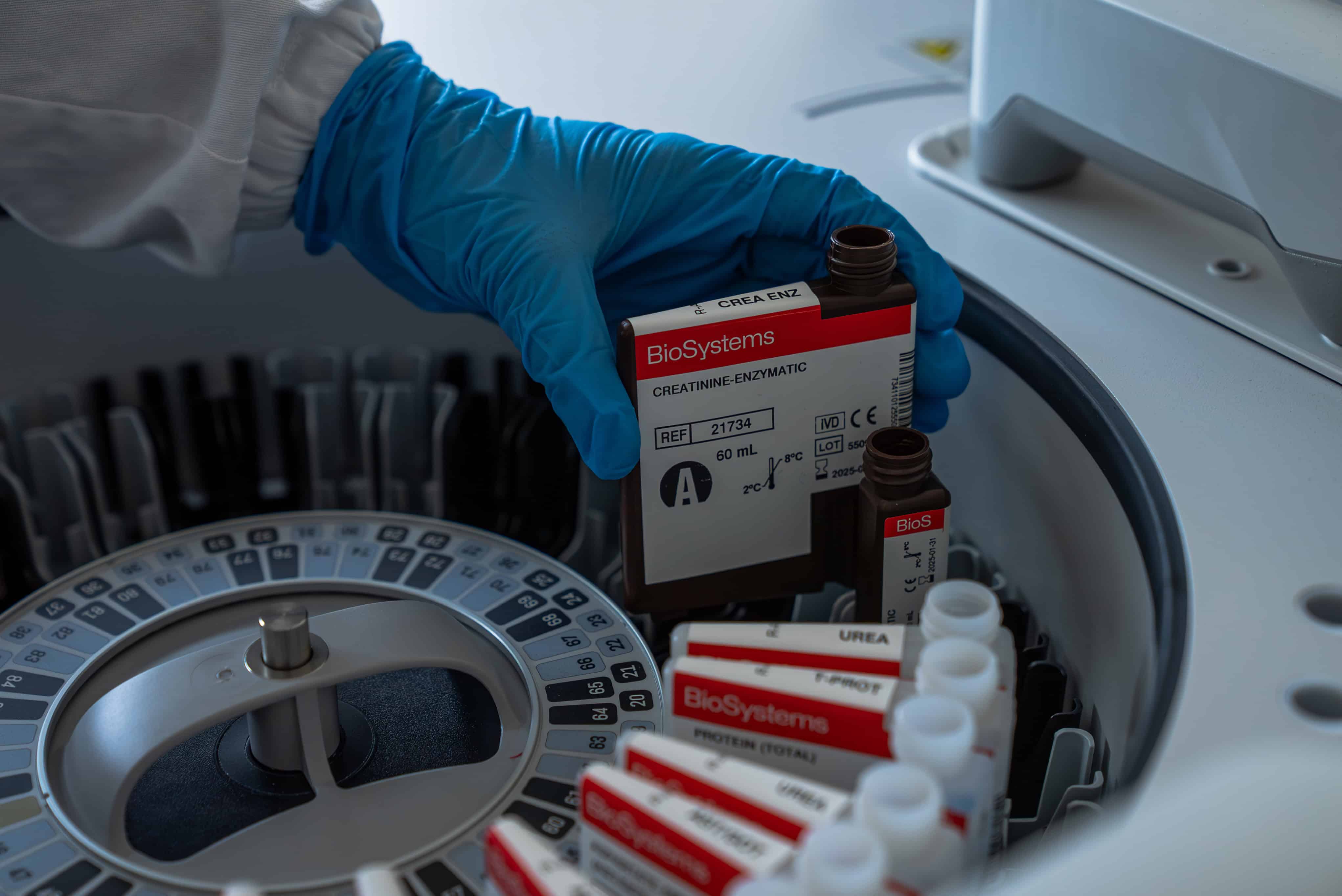
A clinical biochemistry analyser is an instrument that automates the process of analysing liquid biological samples, such as serum, plasma, urine, or cerebrospinal fluid. Analysing a sample means measuring the concentration of certain analytes in it, such as glucose, cholesterol, proteins, or liver enzymes. Knowing these concentrations helps doctors make informed decisions about diagnosing a potential disease in the patient or about its treatment. Do you want to know how it works?
- About
- Solutions
-
Clinical Analysis
-
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Autoimmunity Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Veterinary Analysis
-
Biochemistry Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
Vector-borne Diseases Systems
-
If you are interested in our solutions for Clinical Analysis, you can learn more on our Global website and reach out to us with your needs. Visit our Global Website
-
-
Food & Beverage Analysis
-
Environmental Analysis
-
Bioprocess Analysis
-
- Discover
- Contact
- Resources